Organometallics compounds are those compounds which lie at the interface of organic and inorganic chemistry. Organo-transition metal complexes exhibit a wide range of unique chemical reactions that are not observed in purely organic or inorganic compounds.
Classification of organometallic Reactions
The reactions of organometallic compounds are broadly classified as:
- Ligand Coordination and Dissociation Reactions
- Oxidative Addition and Reductive Elimination Reactions
- Insertion and Elimination Reactions
- Reaction of the Coordinated Ligand
Ligand Coordination and Dissociation Reactions:
Many Reactions of transition metal complexes in solution are followed by either ligand association or dissociation. Some transition metal complexes are prone to substitution that is completed in less than 30 seconds. These reactions are called substitution labile.
Some reactions are completed in more than 30 seconds; these reactions are called substitution inert.
The category of ligand coordination reaction in which associative mechanism is followed by organometallic compounds, the coordination number is increased by one unit at the transition state. It precedes via nucleophilic substitution unimolecular reactions. (SN1)
In SN1 Reactions the mechanism occurs in two steps.
The first is a slow unimolecular process that generates a coordinately unsaturated compound.
The second step is followed by rapid association of a nucleophilic reagent.
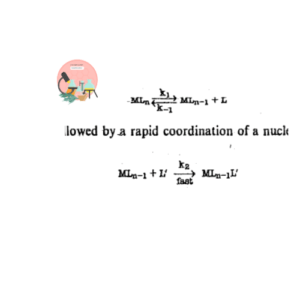
The category of ligand dissociation reactions in which dissociative mechanism is followed by organometallic compounds, the coordination number is decreased by one unit at the transition state. It precedes through nucleophilic substitution bimolecular reactions (SN2)
In these reactions, involving a single step reaction the exchangeable ligands bridged between the two metal atoms either in form of transition state or as an intermediate.
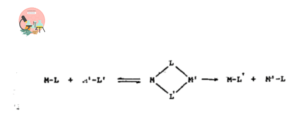
Oxidative Addition and Reductive Elimination Reactions
A typical oxidative addition reaction may be represented as
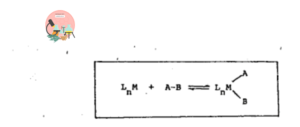
In this reaction the compound A-B adds to the transition metal complex with the breakage of A-B bond.
In Oxidative addition reaction the oxidation state and coordination number of the metal is increased by two units. But in some reactions coordination number and oxidation state can also be increased by one unit is also included in the category of oxidative addition reactions.

Oxidative addition reactions involve various types of bond cleavage:
- Carbon – halogen bond cleavage
- Carbon – hydrogen bond cleavage
- Carbon – oxygen bond cleavage
The reverse process of oxidative addition reaction is called Reductive elimination reaction, both the oxidation state and coordination number are decreased.
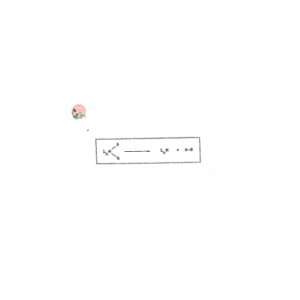
Oxidative addition and reductive elimination reactions are involved in important catalytic reactions in organic synthesis. They were recognized first time in studies of reactions of Vaska’s Complex and Wilkinson’s Catalyst.
Insertion and Deinsertion Reactions
Insertion of an unsaturated compound into metal-carbon or metal-hydrogen bond is very important reaction in many catalytic and stoichiometric reactions. The most important substrates for insertion reactions are carbon monoxide and the olefins.
1. CO Insertion and Deinsertion
Most organometallic compounds of main group elements are not reactive towards monoxide. Many transition metal alkyls are known to react with CO to give transition metal acyls. This reaction is reversible. The reverse process is known as Decarbonylation or Deinsertion or extrusion reaction.
The most obvious requirement for this reaction is that alkyl group and carbon monoxide is mutually situated in cis-position. If they are not in cis position, an isomerization reaction should take place to bring them in cis position otherwise the reaction should not take place.
Carbon monoxide insertion follows by alkyl migration to the coordinated CO ligand in most cases. That’s why this reaction is also known as migratory reaction.
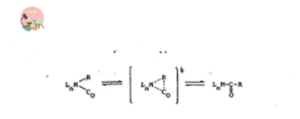
2.Insertion and De-insertion of Olefins
The insertion of olefins in metal-carbon or metal-hydrogen bond constitutes one of the most important catalytic reactions of olefins including hydrogenation, hydroformylation, polymerization reactions. Insertion of olefins in M-H bond is reversible reaction; the reverse of this is beta-elimination reaction. In this reaction olefin is thought to be activated on coordination with transition metal. This activates the M-H bond along with the formation of the product, metal alkyl. It may also be regarded as migratory transfer of hydride ligand to the coordinated olefin same as alkyl migration to the coordinated CO Observed in CO insertion.
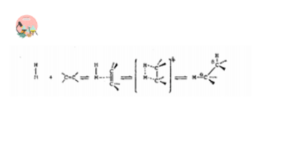
Insertion of CS, CO2, NO, Dienes are also included in insertion and deinsertion reactions.
Reaction of the Coordinated Ligands
-
Reaction of the coordinated Arenes.
The reactivity of the arenes can be changes by complexation to a metal. On completing with metal carbonyl moiety which act as pi-acid electron density on arene is decreased, arene become susceptible for the nucleophilic attack.
-
Reaction of the coordinated CO and Carbene ligands.
In spite of unsaturated nature, carbon monoxide is stable species. The reactivity depends on coordination to transition metal. This causes back donation of electrons from metal to CO, increasing the electron density on oxygen and making carbon relatively electron deficient species. The carbon and oxygen of the Carbonyl become available for the nucleophilic and electrophilic attack.