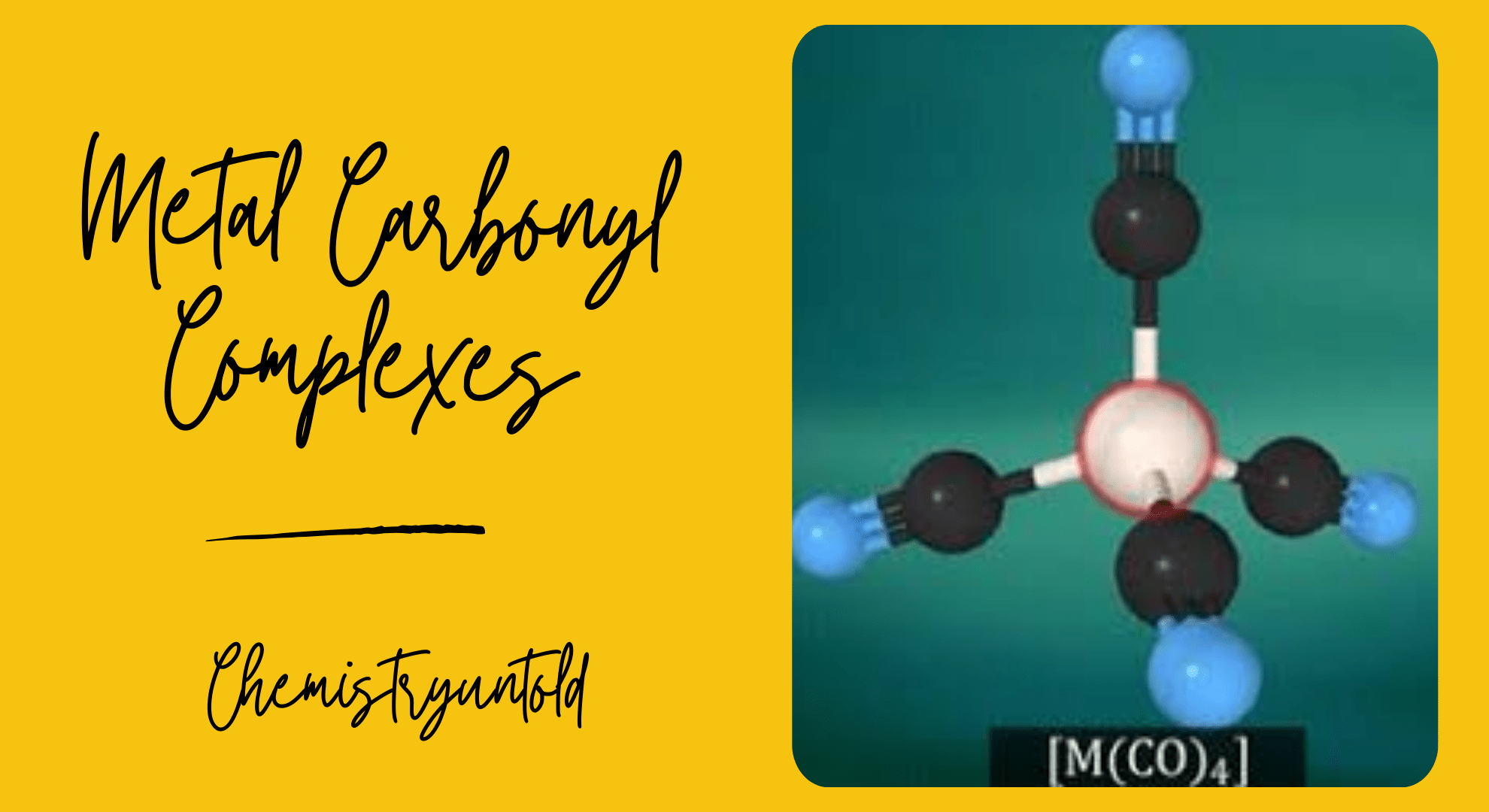
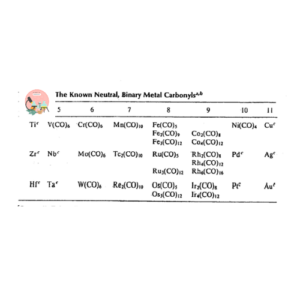
Metal carbonyls are those complexes in which carbon monoxide is a ligand. Metal carbonyls always obey 18-electron rule. Main reason for this is CO is both pi-acceptor and sigma-donor.
Reason
The lone pair on carbon terminal of carbon monoxide ligand donate a lone pair of electron to metal to form coordinate covalent bond. On the other hand CO has empty anti-bonding molecular orbitals which can accept electron density from metal. This back donation of electron density is due to back-bonding phenomena.
HISTORY
In 1890, tetracarbonyl nickel complex was discovered and it leads to versatility of metal carbonyls since then.
Ludwig Mond synthesize Ni (CO)4 by direct reaction of nickel metal with carbon monoxide. This synthesis provides a base for research and discovery of other metal carbonyls. Mond’s method of preparation was used to purify nickel in the late 19th century.
Ni+ CO Ni (CO)4
CARBONYL LIGAND AND BACK BONDING PHENOMENA
Carbon monoxide itself is not a good base but when is act as carbonyl ligand it forms a strong bond with metal because of strong back bonding phenomena. Requirement of back-bonding include that metal must be low-valent, in low oxidation state and the ligand must have empty anti-bonding orbitals for accepting electron density back from the metal which must be compatible in symmetry and orientation.
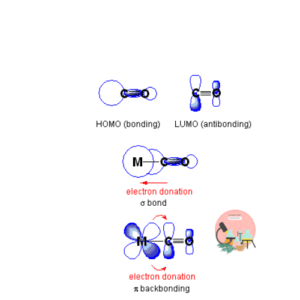
18 ELECTRON RULE (EAN Rule)
The stability of carbonyl complexes is attributed to 18 electron rule. About 90% of the metal carbonyls follow this rule as stable electronic configuration of noble gases. Addition of extra electron is highly unfavorable because extra electron would occupy a higher anti-bonding molecular orbital. And addition of electron in anti-bonding molecular orbital would weaken the M-CO bond.
Likewise, loss of an electron to form 17 electrons would remove an electron from the bonding molecular orbital and weakens the bonding because removal of electron from bonding orbitals eventually weakens the metal ligand bond.
EXCEPTION
Hexacarbonyl Vanadium [V (CO) 6]
One of the exceptions of 18 electron rule is [V (CO) 6]. It is paramagnetic and follows 17 electron rules. Other carbonyls form dimer to compensate the deficiency of 18 electron rule. For example metals like Fe, Ru, Re form dimers as well as other polynuclear complexes. But vanadium cannot form dimer due to following reasons.
- Due to coordination number 7, there will be too much steric hindrance between the ligand which decreases the stability of metal carbonyl complexes.
- Ligand repulsion lead to the weakening of metal-metal bond and it may act as kinetic barrier in dimerization.
V (CO) 6 has ability to accept electron at 70 C to form 18 electron anion.
Na + V (CO)6 Na+ + V (CO)6–
PROPERTIES OF METAL CARBONYLS
- Iron and nickel complexes are liquid at room temperature other metal carbonyls are solid.
- All mononuclear carbonyls are volatile.
- High volatility of Ni (CO)4 along with its high toxicity makes it to be handled carefully. But other carbonyls appear to be less toxic.
- Mononuclear metal carbonyls are non-polar. They are dissolved in hydrocarbon solvent except metal carbonyl of iron [Fe2 (CO)5] because it is insoluble at low vapor pressure.
- Most of the mononuclear metal carbonyls are colorless or have light color. Polynuclear carbonyls are intensely colored. And with increase in number of metal atoms they got even high intensity due to high electronic transitions between orbitals that are largely localized on metallic network.
- Metal centre of simple complexes give reactions like substitution, oxidation, reduction and condensation into large clusters.
SYNTHESIS
MONONUCLEAR METAL CARBONYLS
Mononuclear complexes of Iron and Nickel can be prepared by direct reaction of Carbon monoxide with finely divided metals.
Ni+ CO Ni (CO)4
Fe + CO Fe (CO)5
Mononuclear metal carbonyls can also be prepared by reducing metal halides in presence of CO
CrCl3 + RMgX + CO Cr (CO)6
VCl3 + Na + CO V (CO)6
POLYNUCLEAR METAL CARBONYLS
Binuclear complexes can also prepared by the same method as used for mononuclear complexes.
Re2o7 + 10CO Re2 (CO)10 + 7CO2
Other polynuclear complexes can be prepared by Reduction of a Metal Salt. Reducing agent can be active metals like Al, Na or alkyl aluminum compounds or H2.
Ru (acac)3 + 17CO Re3 (CO)12
ANIONIC METAL CARBONYLS
Anionic metal carbonyls can be prepared by Treatment of Neutral Metal Carbonyls by an alkali and Reaction of metal-metal bonded metal carbonyls.
Fe (CO)5 + 4OH– Fe (CO)42- + CO32- + 2H2O
Mn2 (CO)10 + Na Na+ [Mn (CO)5]–
HYDRIDOCARBONYLS (Carbonyl Hydrides)
Hydridocarbonyls can be prepared by
- Reaction of neutral metal carbonyls with molecular H2
Mn2 (CO)10 + H2 HMn (CO)5
- Acidification of anionic metal carbonyls
Na [Co (CO)4] + H+ CoH (CO)4 + Na
- Reaction of transition metal halides with hydridic reducing agents such as NaBH4
Fe (CO)4I2 + NaBH4 FeH2 (CO)