The VSEPR Theory is a theoretical model that is used to predict the geometry of individual molecules or complexes from the number of electron pair surrounding the central atom. The VESPER theory makes use of positions of different atoms and electrons around the central atom. The final shape of molecule is deduced by ignoring the position of lone electron pair. It is important to note that VESPER Model don’t take steric factor i.e. bulkiness of substituent around the central atom.
HISTORY
- Tangent-Sphere Model
Also known as “spherical electron pair domain Model” developed by Kimball and Bent.
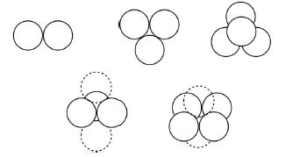
- Sidgwick and Powel Discovery
Sidgwick and Powell, in 1940, proposed these most primitive coordination profiles of two to six electron pair domains which are actually fundamental to the VSEPR model.
They set the stage for the prediction of the molecular geometries. These predictions explained wide range of Molecular geometries but the distortion from ideal structures was still a challenge to solve.
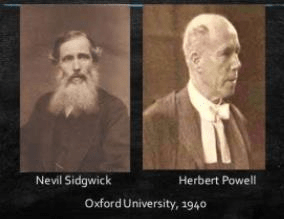
- Gillespie and Nylhom Discovery.
They published their revolutionary paper entitled “Inorganic Stereochemistry”.
They recognized that current model for explaining bond angles did not work well. The problem was that this theory gave incorrect predictions of bond angles for many compounds.


POSTULATES
- If central atom of molecule is surrounded only by bonding electron pairs (bp) not by non-bonding electron pair called lone pairs. Geometry of the molecule will be regular. In the table below Lone pairs are zero
| Number of Bond Pairs | Lone pairs | Geometry | Example |
| 2 | 0 | Linear | Becl2 |
| 3 | 0 | Trigonal Planner | Bcl3 |
| 4 | 0 | Tetrahedral | CH4 |
| 5 | 0 | Trigonal Pyramidal | Pcl5 |
- When the central atom is Surrounded by both Bp’s & LP’s molecule it does not regular geometry.
REASON
Since the lone pair is under influence of only one positive center (Nucleus), they occupy greater electron density than the bond pair which is under influence of two positive centers. The Lone pair is much closer to central atom than the Bond pair so extent more repulsion on any adjacent electron pair. The repulsion between two adjacent Lone pair is maximum in magnitude & repulsion between Lone pair & Bond pair is intermediate while that between Bond pair and Bond pair is minimum.
Lone pair − Lone pair > Lone Pair − Bond Pair > Bond Pair − Bond Pair
Bond pair experience less repulsions from another Bond pair than from a lone pair so bond pair get closer to bond pair & contraction in bond angle occur.
- More the number of lone pair on central atom greater is contraction in Bond Angle. This fact is clear when we compare the Bond angle of CH4, NH3, H2O
| Molecules | CH4 | NH3 | H2O |
| NO. of lone pair on central atom | 0 | 1 | 2 |
| Bond angle (angle between 2 bond pair) | 109.5° | 107.5° | 102° |
| Contraction in Bond angle relative to CH4 | 0° | 109.5-107=2° | 109.5-102=6.5° |
- Bond angle involving multiple bond are generally larger than involving single bond.
Example
CH4 (109.5°) > HCN (180°)
CH4 (109.5°) < C2H4 (120°) < C2H2 (180°)
Electron-Pair Geometry vs. Molecular Geometry:
Electron-pair geometry around a central atom is not the same thing as its molecular structure.
The Electron-Pair geometries describe all regions where electrons are located, bonds as well as lone pairs. The geometry that includes all electron pairs around the central atom.
Molecular geometry describes the location of the atoms, not the electrons. The structure that includes only the placement of the atoms in the molecule
The electron-pair geometries will be the same as the molecular structures when there are no lone electron pairs around the central atom, but they will be different when there are lone pairs present on the central atom
For example
The Ammonia molecule, NH3, also has four electron pairs associated with the nitrogen atom, and thus has tetrahedral electron-pair geometry. One of these regions, however, is a lone pair, which is not included in the molecular structure and the molecular geometry is trigonal pyramid because this lone pair influences the shape of the molecule.
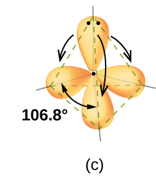
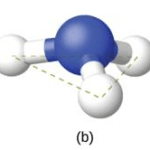
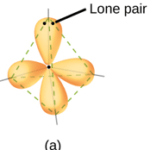
APPLICATIONS OF VSEPR THEORY
VSEPR Theory successfully explained the geometric profiles of molecular species with electron pairs two to six in the valance shell of central atom.
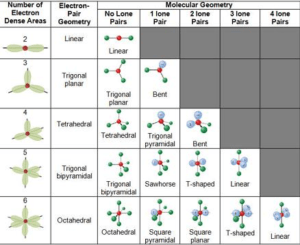
LIMITATIONS
- VSEPR theory has no sound explanation for molecular geometries having polar bonds.
- Theory has no solution for molecular geometries having pi cloud delocalization.
- It fails to predict the structure of transition metal complexes including metal carbonyls. For example many bivalent complexes of nickel are square planner not tetrahedral.
- It fails to explain about isoelectronic species, they may differ in shape despite the same no. of electrons.
- Another limitation of VSEPR theory is that it predicts that halides of group 2 elements will have a linear structure, whereas their actual structure is a bent one.