Ozone (O3) is a low boiling gas. It is present in minute quantities all over the atmosphere. The amount of ozone is expressed in Dobson Units (DU).
Ozone depletion is the major concern now-a-days. The ozone layer extends 12-50km high in the stratospheric region, surrounds the earth and protects the earth from harmful electromagnetic radiations before they could reach the earth. Ozone is produced in most of the tropical regions by photochemical reactions of oxygen, from where it is transported to the other regions.It is also a major component of Photochemical smog (Oxidizing smog), which indirectly effects ozone depletion.
The amount of ozone is less in regions closer to equator. The amount of ozone layer is decreasing over the Antarctic since the mid 1970’s.
“The region in which ozone depletes substantially every year during Sept-Nov is known as Ozone Hole.”
The concentration of ozone is being depleted in stratosphere through various chemical reactions not only Antarctica but globally.
Role of CFCs in Ozone Depletion
Ozone is the main chemical species present in atmosphere which absorbs UV-radiations and increases the temperature in the upper part of the atmosphere. CFCs used as refrigerants in air conditioning and aerosol sprays are unreactive in the troposphere but slowly diffuses in the stratosphere where they are exposed to UV-radiations generating Cl•. ozone depletion how effects the earth’s atmosphere is depicted in Fig.
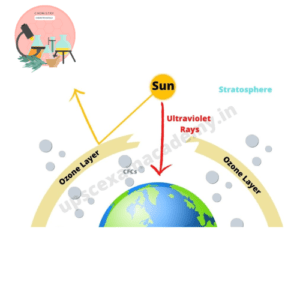
Chloroflourocarbons play an important role in the depletion of ozone in the stratosphere.
CFCl3 →•CFCl2 + •Cl
•Cl + O3→ClO • +O2
ClO + O3 → •Cl + 2O2
Catalytic Ozone Depletion in Stratosphere
Consider reaction of O3 with NO. NO is produced in stratosphere from N2O in trace amount.
NO + O3 → NO2+ O2*
NO concentration is very low w.r.t ozone in the stratosphere. Fraction of O3 destroyed is insignificant if this were the only process taking place. But NO2 produced in this reaction immediately combines with ground state O to regenerate NO.
NO2 + O2 → NO+ O2
Overall reaction is
O3 + O → 2O2
NO is the chain carrier leads to depletion of ozone without being destroyed itself. Mechanism of above reaction can be generalized as follow:
X + O3 → XO + O2*
XO + O2 → X + O2
Overall reaction:
O3 + O → 2O2
Besides NO, X could be H, OH, Cl, Br etc. most significant catalyst in the above reaction is chlorine atom (Cl) in the stratospheric ozone depletion. The primary sources of chlorine atom in the stratosphere are the anthropogenic CFCs. They are also potent greenhouse gases.
Chemically they are very inert and relatively insoluble in water. Because of this inertness and high volatility, they have been found as key industrial uses. Their extreme inertness and insolubility also prevent them from being washed out atmosphere and this is the main reason that they are responsible for ozone destruction in the stratosphere.
CFCs may react with stratospheric oxygen atom to produce reactive substances such as Cl and ClO•. More important is that CFCs absorbs far UV region, photodissociating into chloroflurinated radical and chlorine atom.
CF2Cl2 + hv → CFCl• + Cl
Electromagnetic radiation is prevented from reaching the troposphere by atmospheric oxygen and ozone layer but penetrates in the stratosphere through the UV “window” between 180nm and 220nm. Production of Cl atom is followed by chain mechanism with X = Cl
Cl + O3 → ClO + O2*
ClO + O2 → Cl + O2
Overall: O3 + O → 2O2
Volcanoes(directly involved in Air Pollution) can influence the ozone depletion in ways other than supplying chlorine such as HCl. Volcanic eruption ejects copious quantities debris including SO2. This SO2 is converted to SO3 in stratosphere by OH• and O3. SO3 reacts with gaseous H2O to form H2SO4, producing stratospheric sulphate aerosol of concentrated H2SO4 aerosol droplets small enough to remain in the stratosphere for years.
Enhanced chlorine catalyzed ozone depletion reactions can take place on these aerosols, also causing Air Pollution.
SO2 + OH + M → HOSO2
HOSO2 + O2 → SO2 + H2O
Bromine atom can also take part in catalyzed mechanisms. Bromine atom can come from photolysis of methyl bromide and brominated CFCs (Halons). These CFCs are the direct reason for Ozone depletion.
Methyl bromide naturally occurs in the atmosphere from marine biological activity and forest fires. The anthropogenic halons are widely used in extinguishing fires.
The total concentration of the bromine in the lower stratosphere is almost 19 parts per trillion by volume. Br atoms are 50 times more destructive of ozone than Cl atom on per atom basis, although Cl is overall more destructive because of high concentration of Cl-containing compounds than Br-containing compounds.
Br + O3 → BrO + O2*
Br O + O2 → Br + O2
Overall: O3 + O → 2O2
A factor that quantifies the ability of gas to depletes stratospheric ozone is ozone depletion potential (ODP) which is steady state depletion of ozone calculated for a unit mass of a gas continuously emitted into atmosphere relative to a unit mass of CFC-11