INTRODUCTION
Isomerization is a chemical phenomenon in which a compound or a molecule encounters interconversion in the configuration , proceeding in the generation of one or more isomeric forms. Isomers are molecules with the same molecular formula but different physical and chemical properties or spatial arrangements of their atoms in three-dimensional space.
Isomerization can also indulge various changes in the connectivity of atoms (structural isomerization) or the spatial arrangement of atoms (stereoisomerism).
The process of isomerization is so fascinating and undergo through variety of mechanisms, depending on the specific type of isomerism phenomena and the nature of the atoms that are involved. Some common mechanisms include intramolecular rearrangements, rotation of bond, proton transfer, and transfer of double bond and many more.
The phenomena of Isomerization and the reactions involved are widespread in chemistry and occur in various domains, including inorganic chemistry, organic chemistry, and various industrial processes.
They are very important for selectively synthesizing some specific isomeric compounds, resulting in interconversion of one type of molecule into other type with dversity in characteristics, and comprehending the complex behavior of chemical systems.
Isomerization vs Isomerism
Isomerization and isomerism are interchangeable conceptions in chemistry, but they pertain to different phenomenas.
Isomerism:
Isomerism is defined as the phenomenon where two or more than two compounds have the same molecular formula but different physical or chemical properties or different structural rearrangements or spatial orientations of atoms. Isomerism can be exhibited in different forms, including stereoisomerism and structural isomerism
- Structural isomerism involves differences in the connectivity of atoms within molecules.
- Stereoisomerism involves differences in the spatial arrangement of atoms.
Isomerization:
Isomerization is a chemical phenomena where one compound is transformed into another one having the same molecular formula but a variable structural configuration leading to the formation of different isomeric forms.
This transformation includes the rearrangement of different atoms within the molecule. Isomerization reactions can occur through various mechanisms, such as structural configurations, geometric rearrangements, or stereochemical changes.
Isomerization reactions are often reversible and can be controlled by various parameters such as pressure, temperature and the catalysts.
TYPES OF ISOMERIZATION
Broadly speaking there could be different types of isomerization phenomena. Isomerization can proceed through different mechanisms, depending upon the type of molecules involved and the nature of specific reaction conditions.
Some common types of isomerization reactions are described below
-
Structural Isomerization:
This involves the rearrangement of different atoms within the same molecule having same molecular formula leading to the formation of structural isomers.
Examples
-
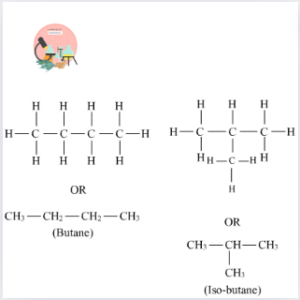
Butane and Isobutane:
Isobutane (C4H10) and Butane (C4H10) are structural isomers of each other. These two configurations have the same molecular formula but different structural configurations. Butane has a linear structure, while isobutane has a branched configuration.
-
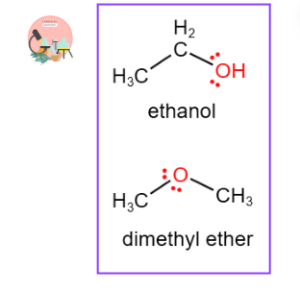
Ethanol and Dimethyl Ether:
Dimethyl ether (C2H5OH) and Ethanol and (CH3OCH3) are structural isomeric forms of each other. Ethanol consists of hydroxyl group attached to a carbon atom, while dimethyl ether has an oxygen atom connected to two methyl groups, showing these two isomers have same molecular formula.
-
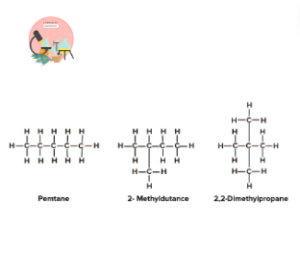
Pentane Isomers:
Pentane (𝐶5𝐻12 ) has three isomeric forms that differ in structure. n-pentane, isopentane, and neopentane.
These differ in rearrangement of the chain of carbon atoms while having same molecular formula.
-
Aldehyde and Ketone Isomers:
Chemical Compounds with the same molecular formula but different functional groups can also exhibit structural isomerism. Functional isomerism is also a type of structural isomerism.
For example, Butanal (𝐶𝐻3𝐶𝐻2𝐶𝐻2𝐶𝐻𝑂) and Propanone (acetone, 𝐶𝐻3𝐶𝑂𝐶𝐻3) are structural isomers.
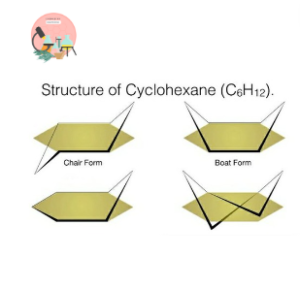
- Cyclohexane Isomers:
Cyclohexane (𝐶6𝐻12 ) occur in various structural isomeric forms, such as chair, boat, and twisted-boat configurations.
-
Geometric Isomerization:
Geometric isomerization, also refers to cis-trans isomerization, is the phenomena in which geometric isomeric forms (chemical compounds having same molecular formula and connectivity of different atoms but differ in spatial orientations around a double bond or a pi-electronic cloud in a ring system) interconvert.
This phenomena is significant in variety of contexts due to its effect on the various physical and chemical characteristics of molecules.
Geometric isomerization only occurs where the rotation around the double bond or cyclic ring system is restricted. It does not occur around single bond.
Key Concepts in Geometric Isomerization
-
Geometric Isomers (Cis-Trans Isomers):
- Cis Isomers: The two atoms or group of atoms are on the same side of the double bond or pi-electronic cloud in ring system.
- Trans Isomers: The two atom or group of atoms are on opposite sides of the pi-electron system.
Examples:
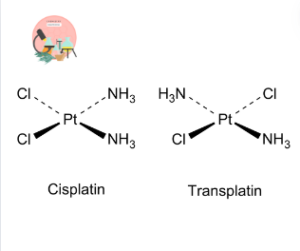
Cisplatin (cis-[Pt(NH₃)₂Cl₂]):
In Cis-platin, the two Chloride are on the same side adjacent to each other. Cis-platin has also significant importance in biological appliactions. It is used as anti-cancerous drug.
Transplatin (trans-[Pt(NH₃)₂Cl₂]):
In Trans-platin the two chloride are on the opposite sides.
-
Activation Barrier and Energy:
- The interconversion from one configuration to the other involves overcoming an energy barrier, which usually requires adequate energy input externally like light or heat. This energy barrier is due to double bonds or strain in the ring of cyclic compounds.
- The energy required for transformation of isomers can be given in form of thermal activation or photochemically (using light).
-
Mechanisms of Isomerization phenomena:
- Thermal Isomerization: In thermal isomerization we heat the compound to supply the required energy to overcome the barrier of activation energy.
- Photochemical Isomerization: In photochemical isomerization absorption of light by the compound occurs, which gives the energy necessary to break the π-bond for a moment, permitting the rotation around the single bond before the pi-bond reconstruct.