INTRODUCTION
Phosphoglucose Isomerase (PGI) also known as phosphoglucoisomerase or phosphohexose Isomerase. The word “ase” at end of Isomerase convey us that it is concerned with the category of enzyme. Phosphoglucoisomerase are the enzymes of Isomerase family and it is called so because they are involved in crucial function of Glycolysis and Glyconeogenesis.
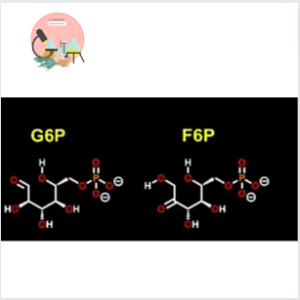
In both of these processes this enzyme is involved in interconversion of Glucose-6-phosphate (G6P) to Fructose-6-phosphate (F6P). This chemical reaction is triggered by appropriate concentration of sugars in the matrix of the cell.
Second step of Glycolysis (one of the most important step of Cellular Respiration) is moderated by Phophoglucose Isomerase. It converts the aldose sugar Glucose-6-phosphate to keto sugar Fructose-6-phosphate.
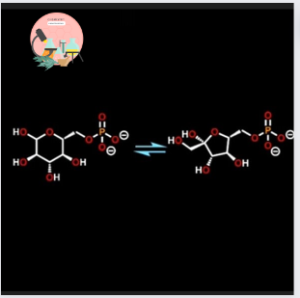
In Glycolysis the molecule act as substrate is Glucose-6-phosphate and it undergoes an Isomerisation reaction forming Fructose-6-phosphate. Isomerization changes the arrangement of the covalent bonds in a molecule but the molecular formula of the isomers remain the same.
∆G for Isomerisation reaction of Glucose-6-phosphate to Fructose-6-phosphate is – 2.92 kj/mole.
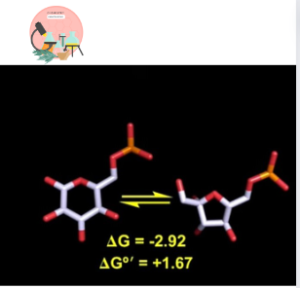
Glucose-6-phosphate ⇌ Fructose-6-phosphate
And it’s ∆G°’ calculated to be 1.67. It means that Isomerization reaction is much closer to equilibrium because ∆G°’ and ∆G both are hovering close to zero.
The reaction above is reversible.
The relative concentration of Glucose-6-phosphate and Fructose-6-phosphate in the cytoplasmic matrix of the cell will determine whether the enzyme will catalyze the reaction in forward or reverse reaction so this enzyme is important in both glycolysis pathway (it is the first important step of Cellular respiration) which breakdown glucose sugar to produce energy and gluconeogenesis pathway which make glucose from non-carbohydrates sources.
Active form of Phosphoglucose Isomerase
Active form of Phosphoglucose Isomerase enzyme is a dimer which means two chains of protein come close together to form active enzyme because two distinct polypeptide chains compromising this enzyme structure has quaternary structure which is its speciality because this structure is not possessed by all the proteins. This molecule is highly symmetrical consisting of beta sheets. Each half of the enzyme contain fully functional active site and is bind to inhibitor.
Some of the amino acids residues from one part of the dimer participate in chemical reaction with the second part of the dimer so dimerization is critical to enzyme activity. These interactions are the same weak interactions that holds substrates and inhibitors in active site. These hydrophobic chains cluster together in various interactions.
Reaction Mechanism of Phosphoglucose Isomerase
In this Isomerisation reaction, we are going from 6-membered ring to 5-membered ring.
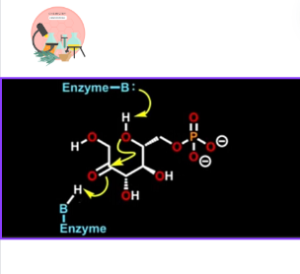
Reaction does not occur on close form of sugar. First of all, enzyme open up the ring, a proton is removed from the hydroxyl group of anomeric carbon using a basic enzyme.
The opening of the ring is facilitated by protonated amino acids within the active site of the enzyme that donate proton to oxygen atom as the ring open up. Since reaction occur on open chain.
Now let’s have a look at Glucose-6-phosphate and Fructose-6-phosphate. We can see that carbonyl group has simply move one carbon atom over.
The Isomerisation step begins when enzyme removes hydrogen atom from carbon next to carbonyl carbon of aldehyde and form enol, a very common intermediate in many biochemical reactions.
Basically this intermediate is also known as Endiol because it has alkene and two alcoholic functional groups. To complete the reaction basic enzyme residues deprotonate alcohol and electron left behind form a carbonyl.
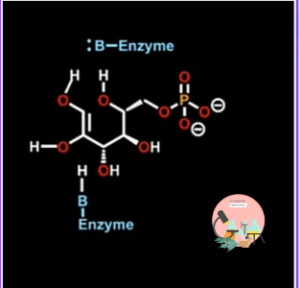
The sugar ring can again close before the product released from the active site. Over all structure of the human and rabbit form of this enzyme is very similar.
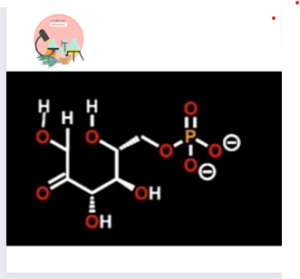
Active site amino acids are completely conserved. Even active site of bacterial Phosphoglucose Isomerase contain identical amino acids to the human form.
In Gluconeogenesis, cyclic form of Fructose-6-phosphate is the substrate of the reaction. For reaction to occur the ring first be opened.
Specificity of active site is dependent upon the amino acids and subtrates nearby.
Many acidic and basic amino acid residues can switch from being protonated and deprotonated to promote the catalysis. Crystallographers that solved this crystal structure proposed that lysine being positively charged is hydrogen bonded to hydrogen atom of the water molecule. this allow water to behave like more hydroxide ion and be a better base.
Since it’s other hydrogen atom is tied up with lysine nitrogen becoming positive charge formally. While oxygen atom of water deprotonates the hydrogen on hydroxyl group of sugar.
This allow the ring to open up with the help of histidine residue
There is another compound similar to Glucose-6-Phosphate, 6-phosphogluconic acid. Both compounds have 6 carbon atoms, phosphate group and a carbonyl group but the major difference is that that 6-phosphogluconic acid contain carboxylic acid which is negatively charged at the biological pH instead of aldehyde that Glucose-6-Phosphate has.

Carboxylic acid groups prevents the compound from forming the ring and give more information how phosphoglucose Isomerase interact with the linear substrate.