The word bleach is a generic term that is used for any chemical substance that is used to remove colors from fibers or different materials.
So in short, process of removing colors or making it lighter is bleaching. Bleach come in different forms; there are bleaching powders, liquid bleach, bleach gel, wipes or spray depending upon how you are going to use it.
Composition of Bleach
The active ingredient in bleach in NaClO, aqueous solution of sodium hypochlorite is bleach. Bleach is an active cleaning agent first introduced in 1785 by Claude Louis Bertholle.
Is Bleach Acidic or Basic
The criteria of assigning a chemical compound an acid or a base is dependent on whether its lie in Acidic or Basic pH.
And also there are various theories which make us clear and give information whether a particular substance is acid or a base. So, these two parameters one is the pH scale and the other are theories which help us determining the nature of bleach which is commonly used in houses.
Before describing these theories let us have a look at Chemical formula of Bleach.
Chemical formula of Bleach
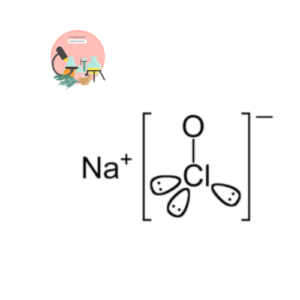
It consist of two ions sodium ion (Na+) and hypochlorite ion (ClO–).
The sodium ion generated after ionization of sodium hypochlorite is neutral and hypochlorite ion has an unshared pair of electron.
NaClO → Na+ + ClO–
Theories regarding Acid and Base
Arrhenius theory
This theory defines that acid is the one that give hydrogen ions in aqueous solution and base is the substance that give hydroxide ions in aqueous solution.
(Acid)H2SO4 → H + SO4
(Base) NaOH → Na+ + OH–
Lowry-Bronsted Concept
According to this theory acid is substance that donate protons and base is a substance that accept proton.
In short acid is a proton donor and base is a proton acceptor.
CH3COOH (Acid) + H2O (Base) → CH3COO– + H3O+
Lewis Concept
According to Lewis concept acid is a substance that is electron acceptor and base is the one that is electron pair donor having unshared pair of electron donated to other molecules.
Now let have a look at Sodium hypochlorite in respect of these theories this equation written above can be rewritten as
NaClO → Na+ + ClO–
Sodium ion release in this reaction is neutral is neutral in nature and does not predict the nature of a solution whereas hypochlorite ion act as electron donor having unshared paired of electron so it act as a base according to Lewis concept like wise hypochlorite ion is also a proton accepted having an ability to accept proton so it’s a base according to Lowry- bronsted concept according to Arrhenius concept hypochlorite ion give hydroxide ion in aqueous solution This molecule also fullfills the criteria of being a base according to Arrhenius concept.
ClO– + H2O → HClO + OH–
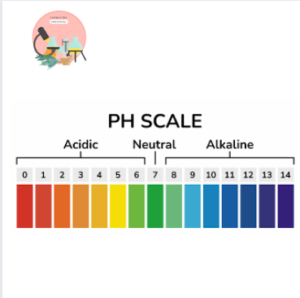
pH scale for designing Acidic or Basic nature of Bleach
- if the pH is 7 the substance is neutral
- if the pH is less than 7 the substance is acidic.
- if the pH is greater than 7 the substance is basic.
The bleach sodium hypochlorite solution is basic in nature its pH is greater than 7 it can be explained with an example
Example :
Calculate the pH of 0.01M solution of NaClO?
First we have to determine its Kb value that describes basic strength of a compound.
As we know Ka of solution (provided in literature) is 3*108.
Ka*Kb = 10-14
Kb = 10-14 / 3*108
= 3.3*10-7
Kb = [ClO–] [OH–]/ NaClO – [ClO–]
Assume that
[ClO–] = [OH–] = X
3.3*10-7 = X2/ 0.01-X
[OH-=] = 1.8*10-4
pOH of solution is calculated as follow
pOH = -log[OH–]
= -log(1.8*10-4)
pOH = 3.7
We know that
pH + pOH = 14
pH = 14-3.7
pH = 10.3
pH of bleach solution is greater than 7 hence, bleach is basic in nature.
Conclusions
pH of bleach is between 8-12, it’s greater than 7 so it’s a base and also fits in definition of bas according to above mentioned theories.
How bleach affect the pH in Swimming pools
People use bleach because it’s a cheap way to chlorinate pool and reduce algae.
One of the side effects of adding bleach in pool, it increases the pH value. Initially when you add bleach to the water it has a pH of around 11-12 and it will increase the pH.

When your water contain algae adding bleach results in oxidation of algae lowering of pH occur This happens if you add bleach for a short time but if bleach is added for a longer period of time it may cause many problems
When it oxidizes algae and decreases the pH it has a net effect of not affecting the pH at all however some of the bleach brands at stabilizing agents which result in increase in pH and it likely results in algae precipitation of minerals from water and lack of efficiency with chlorine at oxidizing algae. Read more…