Hydrocarbons are the class of Organic compounds which contain carbon and hydrogen only. Aliphatic hydrocarbons are divided into two classes. There is variety of organic compounds because of property of Catenation.
Saturated Hydrocarbons
The compounds in which all the four vacancies are fully utilized by single bond are called saturated hydrocarbons. Alkanes are included in this category.
Unsaturated Hydrocarbons
The hydrocarbons in which there is double or triple bond between two carbons are unsaturated hydrocarbons. Double bonded hydrocarbons are Alkenes and if there is triple bond between two carbons are Alkynes.
ALKANES (Saturated Hydrocarbons)
Alkanes are also known as paraffins (“para” means little, “affins” means affinity). Simplest member of this family is Methane (CH4), having general formula is CnH2n+2 containing carbon and hydrogen only. Each carbon is sp3 hybridized.
PREPARATION METHODS
Hydrogenation of Unsaturated Hydrocarbons/ Sabatier-Senderns Reaction
Hydrogenation of alkenes and alkynes in presence of Ni at 200-300°C result in formation of Alkanes. This reaction also occurs in presence of Pt and Pd, but these are expensive.
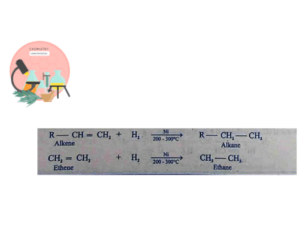
This reaction is also known as Sabatier-Senderns reaction after the name of two scientists Sabatier and Senderns and were given Noble Price for their discovery.
Industrial Importance
Production of vegetable ghee by catalytic hydrogenation of vegetable oil is example of application of this method on Commercial scale.
Vegetable Oil + H2 → Vegetable Oil
From Alkyl Halides
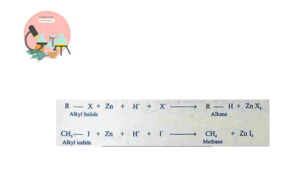
Alkanes are also produced from alkyl halides when it reacts with Zn metal in presence of an aqeuos acid.
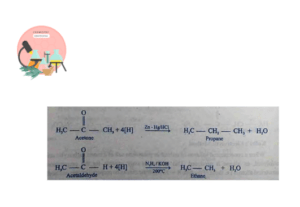
From Carbonyl Compounds (Aldehydes and Ketones)
Alkanes can be prepared from Carbonyl compounds. Carbonyl group of aldehydes is reduced to Methyl group by Wolf-Kishnner Reduction method. Aldehydes are reduced to alkanes with KOH in presence of Hydrazine at 200°C. In case of ketones, Carbonyl group is reduced to methylene group by Clemenson’s Reduction. Ketones are reduced to saturated hydrocarbons using zinc amalgam and HCl.
From Grignard Reagent
Grignard Reagent is important class of compounds discovered by Victor Grignard in 1900. All classes of organic compounds can be prepared using Grignard reagent. They are formed when Alkyl Halides react with Magnesium in presence of anhydrous ether.
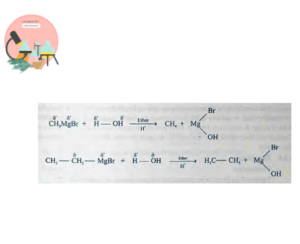
They decompose on treatment with water or dilute acid to give alkanes.
REACTIVITY OF ALKANES
Alkanes or paraffins (means little affinity) are inert towards acid or alkalies under ordinary conditions. They undergo two types of reactions.
- Substitution reactions
- Thermal or catalytic reactions
The unreactivity of alkanes is due to two reasons.
Non-Polarity
Electronegativity values of Carbon (2.5) and Hydrogen (2.1) do not differ significantly and bonding electrons are equally shared between two atom in a bond formation so attacking reagent don’t find any site for reaction.
Inertness of sigma-bond
In δ-bond electrons are tightly held which makes it a very stable bond. A lot of energy is required to break this bond. These electrons neither attack on electrophile nor nucleophile attack on them.
ALKENES (Unsaturated Hydrocarbons)
Alkenes are also known as olefins because lower members of this family form oily products on reaction with halogens. The simplest olefin is C2H4 having general formula CnH2n.
PREPARATION METHODS
Dehydrohalogenation of Alkyl Halides
Alkyl Halides on heating with Alcoholic Potassium Hydroxide undergo dehydrohalogenation i.e. elimination of hydrogen and halogen from adjacent carbon atoms.

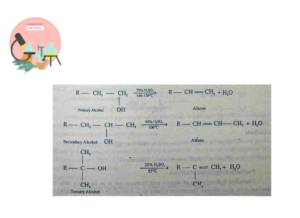
Dehydration of Alcohols
Alcohols when dehydrated in presence of catalyst give alkenes.
P4O10, H2SO4 and H3PO4 are also used for dehydration. Ease of dehydration for PPrimary, Secondary and Tertiary alcohols is
Ter. Alcohol > Sec. Alcohol > Pri. Alcohol
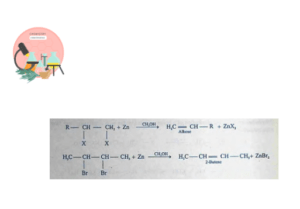
Dehalogenation of Vicinal Dihalides
Vicinal dihalides have two halogens on adjacent carbon atoms. Dehalogenation occurs when dihalides are treated is treated with Zn dust in any anhydrous solvent like methanol or acetic acid.
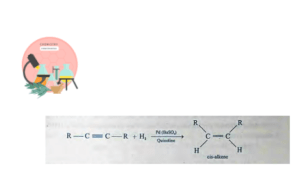
Partial hydrogenation of Alkynes (Controlled synthesis)
Controlled hydrogenation of unsaturated hydrocarbons (alkynes) with hydrogen gas in equimolar ratio over heated catalyst, give alkenes. Catalyst is finely divided Pd supported on BaSO4 and poisoned by treatment with quinoline (Lindlar’s catalyst).
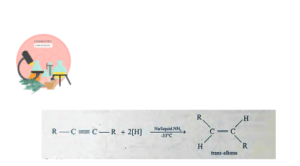
A trans-alkenes is produced by treating alkyne with Na in liquid NH3 at -33°C. This reaction also shows Regiospecificity.
REACTIVITY OF ALKENES (Unsaturated Hydrocarbons)
Reactivity of alkenes is due to presence of pi-bond , the partially-filled p-orbitals overlap in parallel fashion. -bond is less firmly held between the two nuclei. -bond is weaker than sigma-bond and is more exposed to attack by electrophilic reagents. Alkenes undergo electrophilic reactions very easily.
ALKYNES (Unsaturated Hydrocarbons)
Unsaturated hydrocarbons having triple bond between two carbon atoms. Acetylene(C2H2) is the simplest member of this family having general formula CnH2n-2.
PREPARATION METHODS
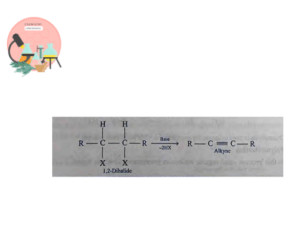
Dehydrohalogenation of Vicinal Dihalides
Vicinal dihalides on treating with strong base eliminates two molecules of hydrogen halides from adjacent carbons to give alkyne.
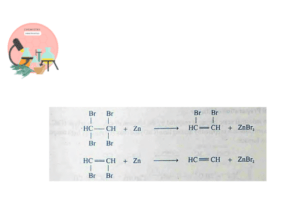
Dehalogenation of Tetrahalides
Tetrahaloalkanes on treatment with active metals like Zn, Mg etc. form alkynes.
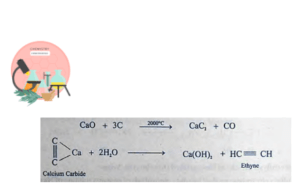
Preparation of Acetylene on Commercial Scale
On industrial scale ethyne is prepared by the reaction of CaC2 with water. Calcium Carbide is prepared by heating lime (CaO) with Coke (C) at very high temperature in electric furnace.