Electrode potential literally means potential of an electrode. We know that each cell is made up of two electrodes. One electrode is the cathode at which reduction occurs and the other electrode is anode where oxidation occurs. For example in electrochemical cells. oxidation occurs on Zinc electrode and reduction occurs at copper electrode.
The tendency of an electrode to oxidize or reduce when come in contact with the solution of its own ions is called electrode potential. So it’s measure of tendency of an electrode.
Oxidation and Reduction(redox) Potential :
The cell reaction consists of two reactions, one is the oxidation reaction and other is the reduction reaction. Cell may be regarded as made up of two single electrodes. Their algebraic sum equals to the electromotive (emf) of a cell.
Ecell = Eanode + Ecathode
Eanode is the measure of tendency of anode to lose electron and undergo oxidation.
M → Mn+ + e–
Whereas the Eanode is the oxidation potential, denoted by Eoxi
Ecathode is a measure of tendency of cathode to gain electron and undergo reduction.
Mn+ + ne– → M
Whereas, Ecathode is the reduction potential denoted by Ered. Since the cell reaction is a redox reaction can essentially be written as
Ecell = Eoxi + Ered
Standard Electrode Potential
Defined as potential of an electrode when electrode is in contact with solution of its ions and concentration is 1M at 25 °C and 1atm pressure. It is signified as E°.
Nernst Theory :
It is well known that ultimate source of emf in the galvanic cell is the chemical reaction which proceeds when the current.
In 1889, Nernst proposed a theory to explain the above phenomena. According to this theory all the metallic elements and hydrogen have a tendency to pass in solution in the form of ions.
This property of metal is known as solution pressure or solution tension of the metal and it is constant at a given temperature. If the zinc is immersed in pure water, zinc ions pass into the water under the stress of solution pressure of the metal.
Zn → Zn2+ +2e–
Zinc metal is left with the negative charge due to migration of positive ions away from it. Hence, there exist a potential between electrode and electrolyte. On account of large magnitude of the charge involved leads to the formation of electric double layer. Thus, the formation of electric double layer prevents the further expulsion of ions from the metal and there is rapidly established equilibrium with a definite potential difference.
If the metallic electrode is dipped in solution of its salts, the position becomes slightly different. In such case the tendency of ions is to be deposited on electrode. This backward reaction is attributed to the osmotic pressure of ions in solution.
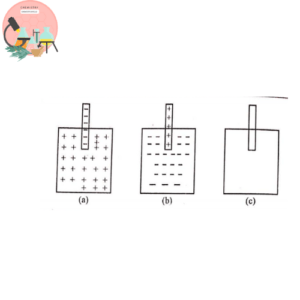
Thus we can say standard electrode potential is equal to potential difference between its solution pressure and osmotic pressure. There arise three possibilities.
- If the solution pressure is greater than osmotic pressure, the tendency of metal to lose ions predominate, a potential difference is set up left the metal with the negative charge w.r.t solution. For example: Zn, Mn, Cd and alkali metals. Fig (a)
- If the solution pressure is less than the osmotic pressure, a positive charge will be developed on the metal and ions will leave the solution to get deposited on the electrode. Examples are Cu, Ag, Au, Hg etc. Fig (b)
- When solution pressure is equivalent to osmotic pressure, no relative charge is developed and no potential difference exists. Such systems are sometimes called null electrodes. Fig (c)
Measurement of Electrode Potential
There is no way to measure the electrode potential of single cell of an isolated half-cell. We can only measure when two half cells are connected. It is mandatory to couple an electrode with reference electrode. The potential of a reference electrode is always taken as zero.
Two reference electrodes are described here.
1. Standard Hydrogen Electrode (SHE)
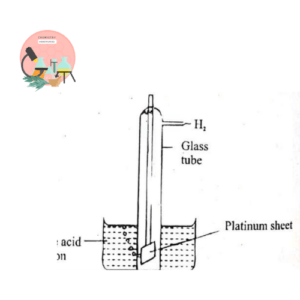
Gaseous hydrogen at 1atm is bubbled over the Pt electrode coated with finely divided Pt which causes electrode reaction to occurs rapidly. The electrode is present in dilute acid solution at 25°C and H+ concentration is 1M as shown in the Fig below.
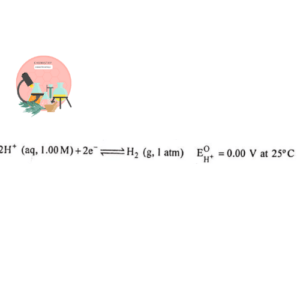
The electrode potential of H2 is arbitrarily taken as zero. The potential of all other electrodes are measured with reference to hydrogen scale obtained by combining SHE with other electrode.
2. Calomel Electrode
This electrode is mostly used in laboratory. It consists of glass vessel with small quantity of pure mercury at the bottom, the Hg is covered with paste of mercurous chloride is filled with solution of saturated Kcl.
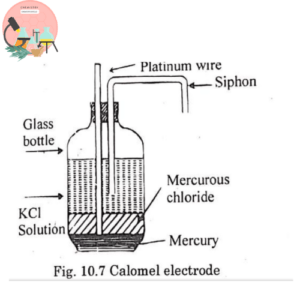
The Pt wire is fused in glass tube and siphon is provided for connection with electrode whose potential is to be measured.